PhD Program of Study Progress
- Scheduling Quals for the Spring
- To Do – email committee with update and to schedule date
Chapter 2: Invasion history of X. crassiusculus
- Library containing the following individuals sequenced.
- To Do – Analyze data!
-
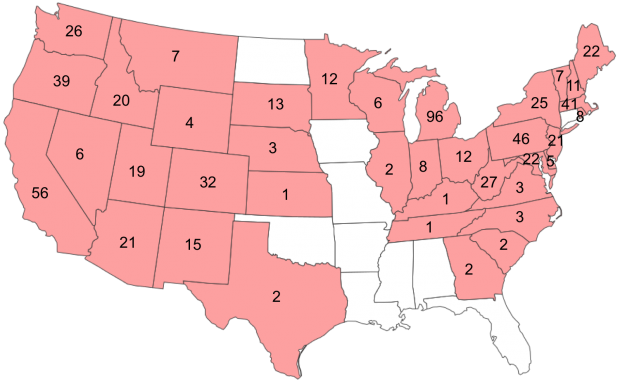
Brunei 1 Cameroon 8 China 20 Ghana 12 Ankasa 9 Kakum 3 Honduras 3 Indonesia 12 Japan 20 Aichi 6 Iriomote Is. 2 Okinawa 12 Madagascar 8 Malaysia 2 New Guinea 7 Taiwan 7 Thailand 4 USA 111 AR 5 FL 24 GA 23 Hawaii 7 MD 8 NC 12 SC 12 VA 20 Grand Total 215
Chapter 3: What is a species? Molecular and morphological delineation of the X. ferrugineus/bispinatus species complex
- Begin with measurements of simple characters of a few practice specimens. For example, width, length, elytra, and color
- To Do – practice mounting elytra
- To Do – Finalize specimen list
- To Do – Plan collecting trip for the Spring?
Chapter 4: Invasion history of an outbreeding invader D. valens
- Follow up on acquisition of specimens
- Finalize specimen numbers
- Begin dissections